Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá Oct./Dec. 2019
https://doi.org/10.22516/25007440.260
Case report
A case report of malignant gastrointestinal melanoma of unknown primary origin
1Especialista en Medicina Interna; fellow en Gastroenterología, Universidad Javeriana, Universidad del Rosario; docente Universidad de Nariño; Gastroenteróloga Fundación Hospital San Pedro, Nariño, Colombia
2Médico General, Facultad Ciencias de la Salud, Universidad de Nariño, Pasto Nariño, Colombia
One of the unusual characteristics of cutaneous melanoma is its ability to metastasize in the small intestine. It is often diagnosed during autopsies of cutaneous melanoma patients. Metastatic deposits have been found in 50% to 60% of these autopsies, but less than 2% to 4% of patients diagnosed with melanoma have gastrointestinal metastasis during the course of the disease. Between 4% and 9% of gastrointestinal melanoma cases have unknown primary tumors.
Rapid identification and resection of melanoma in the digestive tract could improve the patient survival rate and prevent complications such as intestinal obstructions from occurring. We present a rare clinical case of gastrointestinal melanoma of unknown primary origin. The patient had a clinical picture of nausea, hyporexia, epigastralgia, fatigue, paresthesias in the right dorsal region and had lost nine kilograms in three weeks. An abdominal CT scan showed three predominantly isodense liver lesions in the parenchyma, with some areas of lower density located in segments 2,5,7 and 8 of the liver. These were biopsied. Upper digestive tract endoscopy took biopsy samples of two hyperpigmented lesions in the second portion of the duodenum. Histopathological examination showed malignant melanoma. All typical locations of primary melanoma were excluded during the diagnostic procedure.
Keywords: Melanoma; metastasis; unknown primary neoplasms; gastrointestinal tract
Una de las características inusuales del melanoma cutáneo es la capacidad de metastatizar en el intestino delgado. A menudo se diagnostica durante la autopsia, en la cual se ha encontrado depósitos metastásicos en el 50%-60% de los casos, pero menos del 2% al 4% de los pacientes con diagnóstico de melanoma tiene metástasis gastrointestinal (GI) durante el curso de la enfermedad y entre el 4%-9% de casos de melanoma GI tiene un primario desconocido. La rápida identificación y resección del melanoma en el tracto digestivo podría mejorar la tasa de supervivencia en aquellos pacientes en los que se presentan complicaciones, como obstrucción intestinal.
Se presenta un caso clínico de melanoma gastrointestinal de origen primario desconocido. El porcentaje de este tipo de casos es poco frecuente. La paciente presentó cuadro clínico de náuseas, hiporexia, epigastralgia, fatiga, parestesias en región dorsal derecha y disminución de 9 kilogramos en 3 semanas. Le realizaron tomografía computarizada (TAC) de abdomen contrastado, que reportó 3 lesiones en hígado de predominio isodenso en el parénquima, con algunas áreas de menor densidad al interior, localizadas en los segmentos 2, 5, 7 y 8 del hígado al que se le realizó la biopsia. Se llevó a cabo endoscopia de vías digestivas altas con biopsia de 2 lesiones hiperpigmentadas en la segunda porción de duodeno. El examen histopatológico de las biopsias evidenció melanoma maligno. Se excluyeron todas las localizaciones típicas de melanoma primario durante el procedimiento diagnóstico.
Palabras clave: Melanoma; metástasis; neoplasias primarias desconocidas; tracto gastrointestinal
Introduction
Malignant melanomas (MM) are tumors that originate from melanocytes in neuroectodermal cells which are derived from neuroectodermal cells. The primary focus of this cancer is usually diagnosed in the skin, eyeball or anus. Melanomas of unknown primary origin represent 1% to 8% of all cases and are usually diagnosed when patients present clinical symptoms of metastases. 1-3 A definitive diagnosis of melanoma of unknown primary origin requires confirmation of histopathological metastases and exclusion of all possible sites of primary foci. Several theories try to explain the etiology of unknown primary melanomas. 2,3 One posits that spontaneous regression of the primary focus occurs as an effect of the immune system. Another posits a malignant transformation of individual melanocytes which enter a lymph node without formation of a focus within any organ. 2,3 The biological behavior of MM is similar to cases with known locations of the primary focus. 4
Clinically, gastrointestinal malignant melanomas are relatively rare and most frequently metastasize from cutaneous primaries. 5 The incidence of gastrointestinal metastases of unknown primary origin is between 4% to 9% of cases. 6 However, primary melanomas can also originate from certain regions in the gastrointestinal tract including the esophagus, small intestine, rectum and anus. 5 The small intestine has the greatest predilection for the development of metastatic melanoma due to its abundant blood supply. The anus and rectum are the most common sites for primary gastrointestinal melanomas due to the presence of melanocytes. 7,8
Incidence rates of this complex, heterogeneous neoplasm vary by gender, age, ethnic group and region. Every year, approximately 200,000 new cases of MM and 46,500 subsequent deaths are diagnosed worldwide. 9 The approximate global incidence of MM was 2.5/100,000 men and 2.6/100,000 women in 2002. 10 In the last 4 decades incidence has continuous increased throughout the world, especially in regions with white populations. In Latin America, data is scarce. (9) Some previous estimates of incidence in Colombia exist, but there is no central cancer registration system of data covering the entire population of the country. The Cali Population Registry of Cancer is the only one in the country that has been used to calculate the incidence for one specific region. 11,12
Cali’s crude incidence rate for MM in men was 1.6 in 1962-1966 and 3.5 in 2003-2007. Inn women it was 1.0 in 1962-1996 and 3.2 in 2003-2007. 9
A rare clinical case of metastasis of melanoma of unknown primary origin to the small intestine and liver is presented.
Case report
The patient was a 46-year-old woman who came to the emergency department of the Fundación Hospital San Pedro because of nausea, hyporexia, epigastralgia, paresthesia in the right dorsal region, and a loss of 9 kg in 3 weeks. She had a history of chronic gastritis, her sister had died of lung cancer ,and her father had a history of prostate cancer. Two years earlier she had undergone two retinal operations on her left eye. Physical examination found her to be hemodynamically stable, allergic, asthenic, adynamic, and dehydrated. She had amaurosis of her left eye. Her abdomen was slightly distended, had normal abdominal sounds, and experienced pain on palpation of the right upper quadrant but had no signs of peritoneal irritation. Here skin had 2 pedicle lesions on her left forearm and on the right lumbar region of her back. The rest of her skin had no lesions suggestive of melanoma.
Her complete blood count was normal, her serum C-reactive protein (CRP) level was high (15 mg/dL), and electrolytes, renal function, blood glucose and coagulation times were within normal values. Ultrasound of the liver and bile ducts showed multiple focal lesions of possible metastatic origin (Figure 1).
An abdominal CT scan with contrast showed a normal-sized liver with lobulated contour and heterogeneous echogenicity. Three protruding isodense lesions were observed in the parenchyma of segments 5, 7 and 8 with several interior areas of lower density. They measured 149 x 128 mm. There were two other lesions, one with diameter of 51 mm in segment 2, and another whose diameter was 57 mm in segment 7. These findings suggested neoplasia. The rest of the abdominal organs were without alterations (Figure 2).
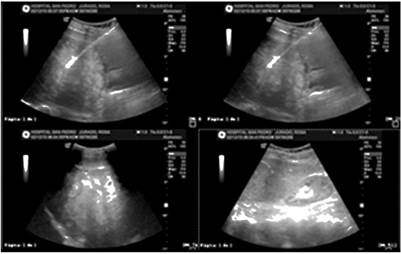
Figure 1 Ultrasound of the liver and bile ducts showing multiple focal lesions of possible metastatic origin.
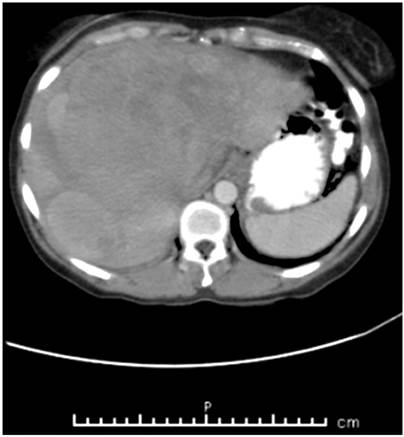
Figure 2 Abdominal CT scan with contrast shows liver with heterogeneous echogenicity, three protruding isodense lesions, with interior areas of lower density inside, located in segments 5, 7 and 8 and two smaller lesions in segments 2 and 7.
Upper endoscopy found antral erythematous gastritis and two hyperpigmented 5 mm lesions in the second portion of the duodenum (Figure 3). Biopsies found malignant proliferations of pleomorphic cells with hyperchromatic nuclei, prominent nucleolus and abundant melanin pigment distributed diffusely throughout the thickness of the mucosa.
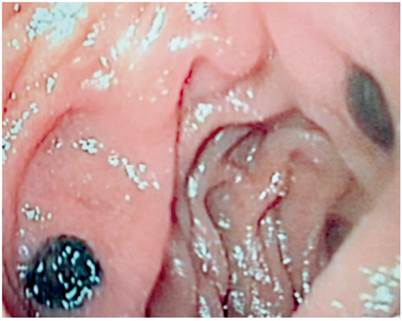
Figure 3 Upper endoscopy. Second segment of duodenum second portion shows two hyperpigmented 5 mm lesions.
The histopathological diagnosis was malignant melanoma. A percutaneous liver mass biopsy identified malignant proliferation of pleomorphic cells with hyperchromatic nuclei, prominent nucleolus and abundant melanin pigment arranged in nests and solid masses.
The patient returned to the emergency department because of her original symptoms plus choluria and hematochezia. Here complete blood count showed a hemoglobin blood count (11.9), slightly elevated liver enzymes (AST: 68 and ALT: 54). Her coagulation times and INR were within normal parameters. A total colonoscopy found grade II internal hemorrhoids but otherwise no abnormalities. Informed consent was obtained for preparation of the case report. The patient is currently undergoing palliative chemotherapy and receiving pain medicine.
Discussion
Malignant melanoma is a tumor that most often occurs in the skin. It occurs less frequently in the eye (choroid layers), leptomeninges, oral cavity, nasal mucosa, pharynx, esophagus, bronchi, vaginal mucosa, rectal mucosa and nails. 11-13 In autopsies, GI metastases have been reported in 50% to 60% of cases, but only 2% to 4% of patients with melanoma are diagnosed with GI metastases during the course of their disease. Primary melanoma in a patient with GI metastasis is most often found in an extremity (15% -57%), followed by the trunk (13% -54%) . They are less frequently found in the head and neck (5% -33%). In 10% to 26% of cases, the primary lesion cannot be found. The period between diagnosis of a primary melanoma and GI metastasis is reported to be up to 54 months. 14 About 70% of patients have symptoms related to the GI tract, while 30% remain clinically asymptomatic. 15
A CT scan is preferred for diagnosis because it can show the most common compromises such as polypoid intraluminal masses, intestinal invagination, ulcerated lesions, diffuse infiltration and implants. 16 The presence of metastatic lesions has been confirmed by surgical exploration in 80% of cases. Eleven percent have been confirmed by endoscopy and 5% by percutaneous biopsies. 15
Metastatic melanoma of unknown primary origin accounts for 1% to 8% of all melanoma diagnoses. The diagnosis is made after exclusion of all possible primary focus locations, as was done with this patient. In 33 years of experience treating melanoma, Savoia et al. found that it was impossible to locate the primary foci in 88 of 4,881 patients. In 31 (35.3%) patients, the first clinical presentation was metastasis in the skin and subcutaneous tissue, in 38 (43.2%) patients it was compromised lymph nodes, while another four patients (4.5%) had compromised lymph nodes and skin. In the remaining 15 patients (17%), melanoma presented visceral metastases. 4
It is very difficult to diagnose primary melanoma of the digestive tract. The suggested diagnostic criteria are absence of another location of melanoma and atypical cutaneous nevi, lesions located only in the small intestine without distant metastases or the presence of a primary focus on the mucosa. Metastases in the liver and duodenum were identified in our patient (Figures 2 and 3) after a detailed analysis of all other possible locations of the primary focus. This could not be identified, therefore, a diagnosis of melanoma of unknown primary origin was made.
When metastasis of melanoma to the digestive tract is suspected, diagnostic images should be taken. The first basic stage of diagnosis is abdominal ultrasound a non-invasive, low-cost exam. Another test is computed tomography, whose sensitivity for identifying melanoma metastases in the digestive tract is only 60% to 70%. In each case, when metastases in the digestive tract are clinically suspected, other tests are necessary when a CT scan is negative. The PET/CT exam has greater sensitivity than CT and should be done if available. 4. In our case, ultrasound and a CT scan revealed metastases in the digestive tract (Figure 1 and 2).
Conclusion
Melanoma of unknown primary origin occurs in 1% to 8% of all diagnosed melanomas. It is usually diagnosed when patients develop clinical symptoms related to metastases such as gastrointestinal bleeding, perforations, or alterations of intestinal transit or absorption. It is relevant to look for the primary focus before making a diagnosis of melanoma of unknown origin. For this, integral evaluation of the patient must take into account the fact that in most cases the primary focus cannot be described.
Recommendations
Complete imaging studies should be done when any patient is diagnosed with malignant melanoma. This will help avoid complications of the underlying metastatic process while making a timely diagnosis of the primary focus (when possible) or arriving at a diagnosis of metastatic melanoma with unknown primary.
REFERENCES
1. Cormier JN, Xing Y, Feng L, Huang X, Davidson L, Gershenwald JE, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer. 2006;106(9):2012-20. doi: doi.org/10.1002/cncr.21835. [ Links ]
2. Giuliano AE, Moseley HS, Morton DL. Clinical aspects of unknown primary melanoma. Ann Surg. 1980;191:98-104. doi: https://doi.org/10.1097/00000658-198001000-00018. [ Links ]
3. Anbari KK, Schuchter LM, Bucky LP, Mick R, Synnestvedt M, Guerry D 4th, et al. Melanoma of unknown primary site: presentation, treatment, and prognosis a single institution study. Cancer. 1997;79:1816-21. doi: https://doi.org/10.1002/(SICI)1097-0142(19970501)79:9<1816::AID-CNCR26>3.0.CO;2-%23. [ Links ]
4. Savoia P, Fava P, Osella-Abate S, Nardò T, Comessatti A, Quaglino P, et al. Melanoma of unknown primary site: A 33-year experience at the Turin Melanoma Centre. Melanoma Res. 2010;20(3):227-32. doi: 10.1097/CMR.0b013e328333bc04. [ Links ]
5. Li WX, Wei Y, Jiang Y, Liu YL, Ren L, Zhong YS, et al. Primary colonic melanoma presenting as ileocecal intussusception: case report and literature review. World J Gastroenterol. 2014;20(28):9626-30. doi: 10.3748/wjg.v20.i28.9626. [ Links ]
6. Patel RB, Vasava NC, Gandhi MB. Acute small bowel obstruction due to intussusception of malignant amelonatic melanoma of the small intestine. BMJ Case Reports. 2012;2012. doi: 10.1136/bcr-2012-006352. [ Links ]
7. Schuchter LM, Green R, Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181-5. doi: 10.1097/00001622-200003000-00014. [ Links ]
8. Alghamdi S, Omarzai Y. Metastatic melanoma presenting as intussusception in an 80-year-old man: a case report. Case Rep Pathol. 2013;2013:672816. doi: 10.1155/2013/672816. [ Links ]
9. Pozzobon F, Acosta A, Carreño A, Fierro E. Características del melanoma cutáneo primario en el Instituto Nacional de Cancerología 2006-2010. Rev Colomb Cancerol. 2013;17(3):111-8. doi: https://doi.org/10.1016/S0123-9015(13)70013-1. [ Links ]
10. Desmond RA, Soong S. Epidemiology of malignant melanoma. Surg Clin North Am. 2003;83:1-29. doi: doi.org/10.1016/S0039-6109(02)00092-0. [ Links ]
11. Nova-Villanueva J, Sánchez-Vanegas G, Porras de Quintana L. Cáncer de piel: perfil epidemiológico de un centro de referencia en Colombia 2003-2005. Rev Salud Púb. 2007;9:595-601. doi: https://doi.org/10.1590/S0124-00642007000400012. [ Links ]
12. Villegas M, Jaramillo F. Comportamiento clínico, epidemiológico e histológico del melanoma maligno en el Departamento de Caldas (Colombia). Rev Asoc Colomb Dermatol. 1999;7:192-6. [ Links ]
13. Woollons A, Derrick EK, Price ML, Darley CR. Gastrointestinal malignant melanoma. Int J Dermatol. 1997;36:129-31. doi: doi.org/10.1016/S0039-6109(02)00092-0. [ Links ]
14. McDermott VG, Low VH, Keogan MT, et al. Malignant melanoma metastatic to the gastrointestinal tract. Am J Roentgenol. 1996;166:809-13. doi: 10.2214/ajr.166.4.8610555. [ Links ]
15. Kawashima A, Fishman EK, Kuhlman JE, et al. CT of malignant melanoma: patterns of small bowel and mesenteric involvement. J Comput Assist Tomogr 1991;15:570-4. doi: https://doi.org/10.1097/00004728-199107000-00008. [ Links ]
16. Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res. 2008;14:638-45. doi: doi.org/10.1158/1078-0432.CCR-07-2025. [ Links ]
17. Blecker D, Abraham S, Furth E, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427-33. doi: 10.1111/j.1572-0241.1999.01604.x. [ Links ]
Received: May 23, 2018; Accepted: July 08, 2018











 text in
text in 


